Abstract
Background: Patients with Sickle Cell Disease (SCD) often require transfusions to minimize disease burden, increase oxygen carrying capacity, and prevent tissue hypoxia. Furthermore, patients with significant complications such as stroke or acute chest syndrome depend on red blood cell exchange transfusion (RBCX) to maintain a decreased percentage of hemoglobin S in circulation and prevent further SCD complications. Red blood cells (RBCs) stored for transfusion develop a storage lesion over time. This is in part due to depleted 2,3-diphosphoglycerate (2,3-DPG) and ATP, which can be reversed by biochemical rejuvenation of RBCs. The FDA-approved rejuvenation process has been shown to normalize hemoglobin oxygen affinity, decrease in-bag hemolysis, and increase RBC deformability. Therefore, we hypothesized that rejuvenation of RBC units prior to RBCX would be associated with diminished loss of hemoglobin A between treatment sessions and increased post-RBCX p50 (the partial pressure of oxygen at 50% hemoglobin saturation, a measure of oxygen affinity).
Methods: Following approval by the Duke University Health System IRB we recruited 4 SCD patients maintained crisis-free on chronic RBCX for the past 3 months. The study had an open-label, crossover design of standard (S) and rejuvenated (R) RBCX sessions in the following sequence: S-R-R-R-S-S. To assess rejuvenation benefit to chronic RBCX SCD patients, we first developed a calculation of perseverance of RBCs given during RBCX using an exponential decay model, and then applied rejuvenation to the final 2-4 donor units given in RBCX, which ensured that approximately 30-40% of post-RBCX circulating RBCs would be rejuvenated. Rejuvenation was performed by trained blood bank personnel according to the rejuvenation solution manufacturer's instructions on RBC units between 14-21 days old. The primary outcome for the study was time-normalized change in Hemoglobin A between RBCX treatments (derived from hemoglobin electrophoresis results), with our secondary outcome being change in p50 before and after RBCX. This study is partially funded by Zimmer Biomet. We report preliminary results demonstrating feasibility of this approach.
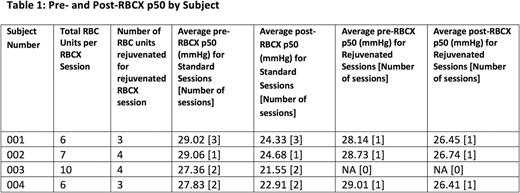
Results: At this time we have completed 8 standard RBCX and 3 rejuvenated RBCX sessions. Preliminary data are presented in Table 1. A total of 10 rejuvenated units have been transfused with no adverse events reported. Despite the low sample size, it was clear that rejuvenation attenuated the p50 decrease seen after RBCX. Hemoglobin electrophoresis data is not yet available as no subjects have had a scheduled RBCX session since their initial rejuvenated RBCX session. RBC rejuvenation was feasible but logistically complicated due to the time and personnel required for the rejuvenation process, and complex coordination between the subjects, the apheresis service, and the blood bank was required for successful implementation. One subject did not present for a scheduled RBCX, resulting in the discard of 4 units of rejuvenated RBCs.
Conclusion: Red cell rejuvenation holds promise as a way of maximizing the exchange transfusion benefit to the most at risk SCD patients. We additionally plan to evaluate change in RBC microparticles before and after standard and rejuvenated RBCX sessions in these patients. Rejuvenation as currently performed provides some technical challenges, such as increased RBC unit processing time and greater required coordination with clinical service.
Landrigan: Zimmer Biomet: Employment, Research Funding. Shah: Novartis: Other: Speaker; Alexion: Other: Speaker. Welsby: Zimmer Biomet: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal